Nada hay en el Mercado para tratar Alzheimer ... y los Farmacos que estan en ensayos caen uno detras de otro ... no hace mucho se publicaba :
*.- Semagacestat . Eli Lilly Suspende los ensayos clinicos para tratar el Alzheimer en plena Fase III .
*.- Dimebon combinado con Aricept para tratar Alzheimer , no termina la Fase III por falta de Eficacia , asi lo han anunciado hoy Pfizer y Medivation .
*.- Medicamentos para tratar Alzheimer que están diseñados para inhibir la BACE1 y que están en ensayos clínicos pueden tener efectos secundarios .
*.- Dimebon en solitario , para Alzheimer de Pfizer no pasa los estudios .
*************************************************+
Grandes Farmaceuticas casi que prefieren ya el no realizar ensayos para Alzheimer y miran de encontrar algun prometedor farmaco en el Mercado que este en ensayos clinicos ... como el caso que hoy se publica .
Roche en el comunicado advierte que hay otros Dos prometedors Farmacos en ensayos Avanzados ( Fase III ) que en breve tambien podrían dar malas noticias .
Alzheimer se esta mostrando muy muy duro de combatir ... son ya 36 Millones los pacientes afectados y se prevee que se duplique la cifra en 20 años ... En EEUU para el 2025 Se predice que la Epidemia de Alzheimer alcanzará proporciones de Crisis.
...
TARLATAMAB TRAS LA APROBACIÓN COMPLETA EN ESTADOS UNIDOS PARA EL TRATAMIENTO SMALL CELL LUNG CANCER EXTENSIVE-STAGE ... AHORA LE TOCA EL TURNO DE CONSEGUIR LA FULL APPROVAL EN EUROPA ... LA EMA PODRÍA DAR SU BENDICIÓN EN LA REUNIÓN DE MARZO .
18 junio 2012
Ecteinascidin - 770 ( ET-770 ) . Molecular network profiling of U373MG human glioblastoma cells following induction of apoptosis by novel marine ...
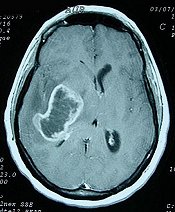 Glioblastoma is the most aggressive form of brain tumors showing resistance to treatment with various chemotherapeutic agents. The most effective way to eradicate glioblastoma requires the concurrent inhibition of multiple signaling pathways and target molecules involved in the progression of glioblastoma. Recently, we obtained a series of 1,2,3,4-tetrahydroisoquinoline alkaloids with potent anti-cancer activities, including ecteinascidin-770 (ET-770; the compound 1a) and renieramycin M (RM; the compound 2a) from Thai marine invertebrates, together with a 2'-N-4"-pyridinecarbonyl derivative of ET-770 (the compound 3). We attempted to characterize the molecular pathways responsible for cytotoxic effects of these compounds on a human glioblastoma cell line U373MG.
Glioblastoma is the most aggressive form of brain tumors showing resistance to treatment with various chemotherapeutic agents. The most effective way to eradicate glioblastoma requires the concurrent inhibition of multiple signaling pathways and target molecules involved in the progression of glioblastoma. Recently, we obtained a series of 1,2,3,4-tetrahydroisoquinoline alkaloids with potent anti-cancer activities, including ecteinascidin-770 (ET-770; the compound 1a) and renieramycin M (RM; the compound 2a) from Thai marine invertebrates, together with a 2'-N-4"-pyridinecarbonyl derivative of ET-770 (the compound 3). We attempted to characterize the molecular pathways responsible for cytotoxic effects of these compounds on a human glioblastoma cell line U373MG....
Doxil . Azaya Therapeutics esta Desarrollando una Versión Genérica de Doxil ( R ) ... El ATI-0918 que se Presenta este año a la FDA .
Azaya Therapeutics ( www.AzayaTherapeutics.com ) is a San Antonio-based emerging biopharmaceutical company whose innovative technology platform allows it to make safer and more effective cancer treatments. Azaya utilizes a proprietary manufacturing process to produce liposome-encapsulated chemotherapeutics that eliminate the use of toxic carriers and incorporates a naturally occurring protein to stabilize the liposomal encapsulated drug. Its unique and patented Protein Stabilized Liposome (PSL(TM)) platform also addresses the significant problems associated with the delivery of water insoluble drugs. Azaya has leveraged this platform to develop a differentiated drug product for the global oncology market with docetaxel as its active ingredient. ATI-1123 is a patented human serum albumin-stabilized nanoparticle liposomal formulation of docetaxel. Docetaxel is available as a generic product and is also the active ingredient in Taxotere(R). Docetaxel is one of the largest selling chemotherapy drugs in the world.
ATI-0918 . The Company is also developing a generic version of Doxil(R), the first liposomal encapsulated chemotherapy drug approved by the FDA. There is currently a global shortage of Doxil due to problems at the manufacturing facility. The Company's management and advisor team is highly experienced in biotechnology and oncology and has a track record of obtaining FDA drug approvals and successfully building companies.
ATI-0918 is undergoing final laboratory studies to establish its pharmaceutical equivalency to DOXIL, and will pursue work as required by the FDA. Due to manufacturing challenges, there is a severe world-wide shortage of DOXIL, and no generic alternative is currently on the market – AZAYA is on a path to resolve this critical problem for cancer patients.
“Doxil currently has global sales over $500 million annually and we will file for FDA approval of ATI-0918 in 2012 to enter this market”.
SOURCE: Azaya Therapeutics, Inc.
ATI-0918 . The Company is also developing a generic version of Doxil(R), the first liposomal encapsulated chemotherapy drug approved by the FDA. There is currently a global shortage of Doxil due to problems at the manufacturing facility. The Company's management and advisor team is highly experienced in biotechnology and oncology and has a track record of obtaining FDA drug approvals and successfully building companies.
ATI-0918 is undergoing final laboratory studies to establish its pharmaceutical equivalency to DOXIL, and will pursue work as required by the FDA. Due to manufacturing challenges, there is a severe world-wide shortage of DOXIL, and no generic alternative is currently on the market – AZAYA is on a path to resolve this critical problem for cancer patients.
“Doxil currently has global sales over $500 million annually and we will file for FDA approval of ATI-0918 in 2012 to enter this market”.
SOURCE: Azaya Therapeutics, Inc.
Una bacteria mutante actúa contra el cáncer de colon .
Una bacteria mutante es capaz de detener una inflamación anormal, reducir tumores precancerosos y revertir el desarrollo de lesiones severas de cáncer en el intestino grueso de ratones, según los hallazgos de un equipo de investigación de la Universidad de Florida, en Estados Unidos.
...
...
Entre 5 y 10% de los tumores cancerígenos son hereditarios .
Asciende al 25 y 33% en el caso del cáncer de colon y mama, respectivamente. Alertan sobre la necesidad de realizar controles para determinar el riesgo real, y así prevenir y curar a tiempo.
...
...
Suscribirse a:
Comentarios (Atom)