Clin Cancer Res. 2017 June .
Ratti C, Botti L, Cancila V, Galvan S, Torselli I, Garofalo C, Manara MC, Bongiovanni L, Valenti CF, Burocchi A, Parenza M, Cappetti B, Sangaletti S, Tripodo C, Scotlandi K, Colombo MP, Chiodoni C.
 Abstract /// Purpose:
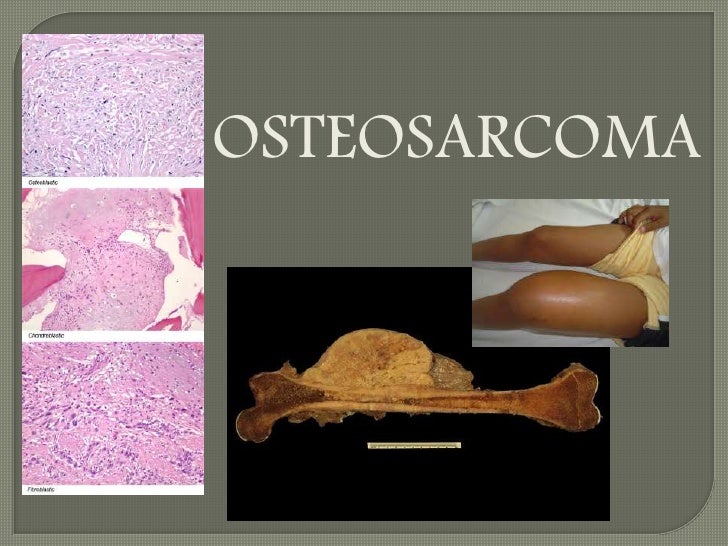
Abstract /// Purpose: Osteosarcoma (OS), the most common primary bone tumor, is characterized by an aggressive behavior with high tendency to develop lung metastases as well as by multiple genetic aberrations that have hindered the development of targeted therapies. New therapeutic approaches are urgently needed; however, novel combinations with immunotherapies and checkpoint inhibitors require suitable preclinical models with intact immune systems to be properly tested.
Experimental Design:
We have developed immuno-competent OS models that grow orthotopically in the bone and spontaneously metastasize to the lungs, mimicking human OS. These models have been used to test the efficacy of trabectedin, a chemotherapeutic drug utilized clinically for sarcomas and ovarian cancer.
Results:
Trabectedin, as monotherapy, significantly inhibited OS primary tumor growth and lung metastases by both targeting neoplastic cells and reprogramming the tumor immune microenvironment. Specifically, trabectedin induced a striking differentiation of tumor cells by favoring the recruitment of Runx2, the master genetic regulator of osteoblastogenesis, on the promoter of genes involved in the physiologic process of terminal osteoblast differentiation. Differentiated neoplastic cells, as expected, showed reduced proliferation rate. Concomitantly, trabectedin enhanced the number of tumor-infiltrating T lymphocytes, with local CD8 T cells, however, likely post-activated or exhausted, as suggested by their high expression of the inhibitory checkpoint molecule PD-1. Accordingly, the combination with a PD-1-blocking antibody significantly increased trabectedin efficacy in controlling OS progression.
Conclusion:
These Results Demonstrate the therapeutic efficacy of trabectedin in OS treatment, unveiling its multiple activities and providing a solid rationale for its combination with immune checkpoint inhibitors.
Copyright ©2017, American Association for Cancer Research.